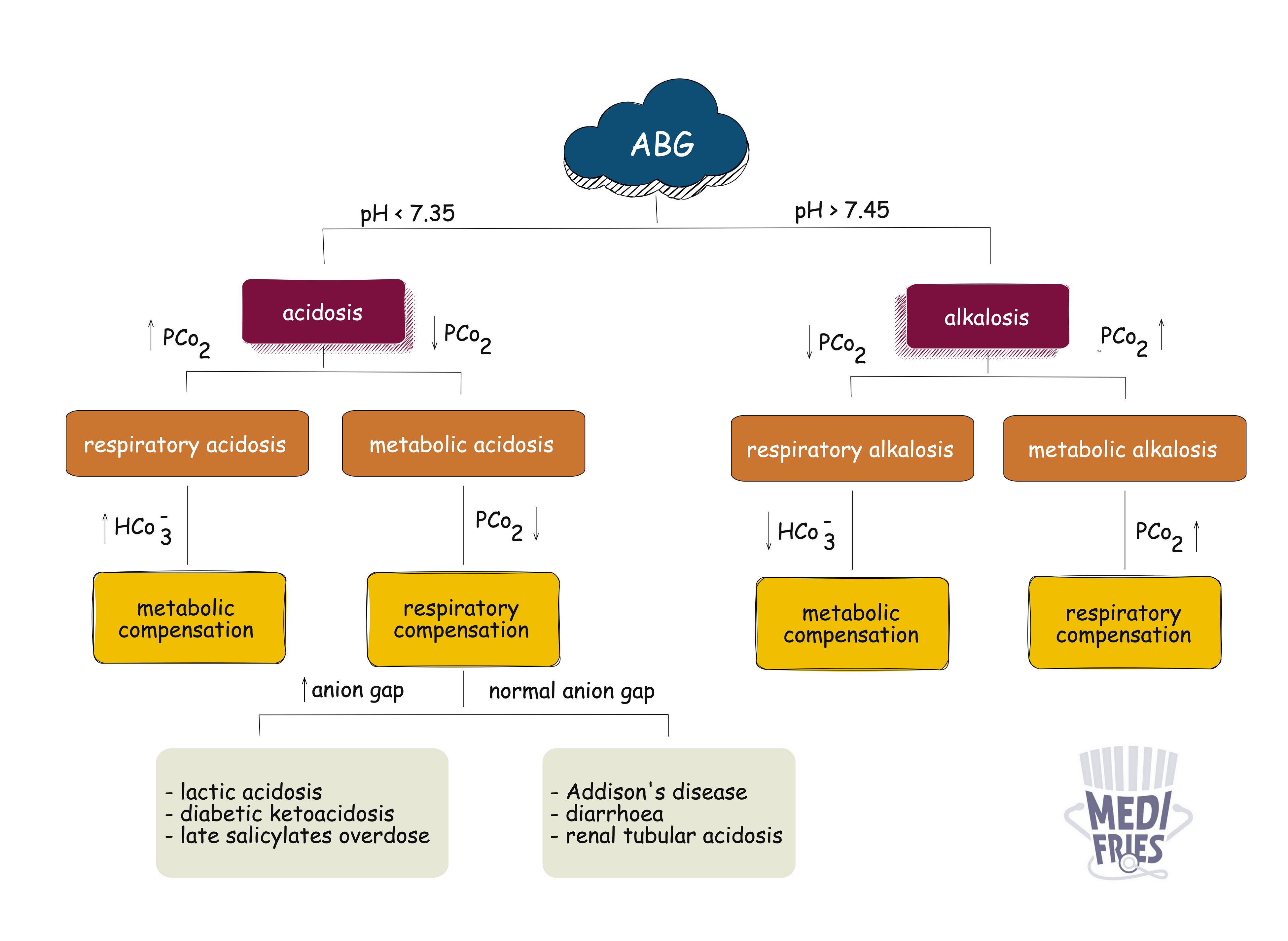
Acid–base disorders refer to disturbances in the body's pH balance due to alterations in hydrogen ion concentration. These imbalances are classified based on pH, PaCO₂, and HCO₃⁻ levels into respiratory or metabolic and acidosis or alkalosis.
| Parameter | Normal Range |
|---|---|
| pH | 7.35 – 7.45 |
| PaCO₂ | 4.5 – 6.0 kPa |
| HCO₃⁻ | 22 – 26 mmol/L |
| Base excess | ±2 mmol/L |
| PaO₂ | 10.5 – 13.5 kPa |
| O₂ saturation | 94 – 98% |
| Disorder | pH | PaCO₂ | HCO₃⁻ |
|---|---|---|---|
| Metabolic acidosis | ↓ | ↓ (comp) | ↓ |
| Metabolic alkalosis | ↑ | ↑ (comp) | ↑ |
| Respiratory acidosis | ↓ | ↑ | ↑ (comp) |
| Respiratory alkalosis | ↑ | ↓ | ↓ (comp) |
Comp = compensatory response
| Cause | Description |
|---|---|
| DKA | Diabetes, starvation, alcohol |
| Lactic acidosis | Sepsis, shock, hypoxia |
| Renal failure | ↓ H⁺ excretion |
| Toxins | Methanol, ethylene glycol, salicylates |
| Cause | Description |
|---|---|
| Diarrhoea | Bicarbonate loss |
| Renal tubular acidosis | Impaired H⁺ secretion |
| Ileostomy/fistula | GI bicarbonate loss |
| Cause | Notes |
|---|---|
| Vomiting / NG suction | HCl loss |
| Diuretics (loop/thiazides) | ↑ renal bicarbonate reabsorption |
| Hypokalaemia | Drives H⁺ into cells |
| Conn's syndrome | Hyperaldosteronism ↑ H⁺ secretion |
| Acute Causes | Chronic Causes |
|---|---|
| Opioid overdose | COPD |
| Airway obstruction | Neuromuscular disease |
| CNS depression | Obesity hypoventilation |
| Cause | Notes |
|---|---|
| Hyperventilation | Anxiety, pain |
| PE, pneumonia | Hypoxia-driven hyperventilation |
| Salicylate overdose | Early phase |
| Pregnancy, high altitude | Progesterone/hypoxia |
Clues:
| Disorder | Compensation Type |
|---|---|
| Metabolic | Respiratory (quick) |
| Respiratory | Renal (slow) |
| Full compensation | Normal pH with abnormal CO₂/HCO₃⁻ |
| Mixed disorder | Inappropriate compensation or conflicting findings |
Fries Tips